登录方式
方式一:
PC端网页:www.rccrc.cn
输入账号密码登录,可将此网址收藏并保存密码方便下次登录
方式二:
手机端网页:www.rccrc.cn
输入账号密码登录,可将此网址添加至手机桌面并保存密码方便下次登录
方式三:
【重症肺言】微信公众号
输入账号密码登录
注:账号具有唯一性,即同一个账号不能在两个地方同时登录。
作者:龚佳红,江叶,钱晓丽
指导:葛慧青
单位:浙江大学医学院附属邵逸夫医院呼吸治疗科
【“VIDD发病机制”部分由龚佳红执笔,“膈肌功能评估”部分由江叶执笔,“预防和治疗”部分由钱晓丽执笔】
【摘要】横膈膜是驱动肺泡通气的主要呼吸肌。越来越多的证据表明,机械通气患者会出现不同程度的膈肌功能障碍。尽管危重症患者救治过程中一系列因素可能会损害危重患者的膈肌功能,但机械通气本身起着至关重要的作用。机械通气部分或完全卸载呼吸肌泵功能,呼吸机过度支持与横膈膜废用性萎缩和肌肉力量丧失有关。在机械通气下保持膈肌活动可能有助于防止萎缩,但是由于呼吸机支持不足或过度呼吸驱动而导致的过度呼吸可能会进一步损害膈肌功能。膈肌功能障碍是直接导致机械通气时间延长以及撤机困难的主要原因之一。因此,如何预防机械通气患者膈肌功能障碍是需要临床关注的问题。
01
1.呼吸驱动和呼吸努力对VIDD的影响
(1)呼吸支持过度
(2)呼吸支持不足
2.呼吸机压力设置对VIDD的影响(PEEP纵向萎缩)
3.人机不同步
4.镇静、镇痛、肌松药物的影响
02
膈肌厚度(diaphragm thickness,DT)是可直接测量的超声指标,包括吸气末厚度和呼气末厚度。健康人群正常膈肌厚度应>2 mm,下限约为1.5 mm,小于2 mm提示膈肌萎缩可能。ICU患者与健康人群的参考值如表1所示。膈肌厚度因身体成分和性别不同而不同[24, 25]。
表1 ICU患者与健康人群膈肌超声参数的参考值[26]
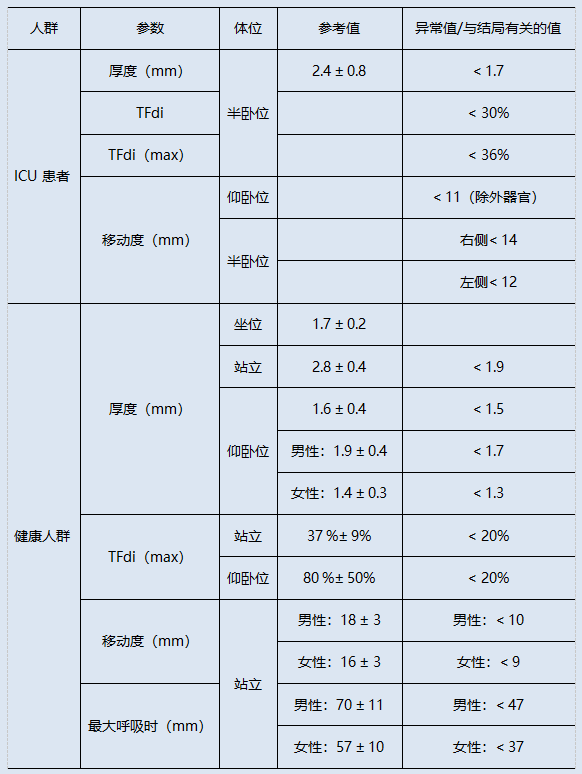
注:电极片位于两侧膈神经的起始部分,在气管插管开口处记录气道压力。白色箭头代表管路断开,全黑箭头代表实施电刺激。隔膜收缩力通过电刺激期间产生的气道压力变化来评估。膈肌功能障碍患者(A)产生的压力下降较小(-8 cmH2O),而膈肌功能正常患者(B)的气道压力正常下降(-30 cmH2O)。
03
膈肌功能障碍是机械通气患者常见和严重的临床问题,我们应重视早期预防,采取积极的治疗措施减少或减缓膈肌功能障碍的发生和发展。
通过选择合适的呼吸支持方式,调整参数设置从而减少人机不同步的发生;并适当减少镇静,保留自主呼吸,改善患者自身呼吸做功,把握时机,积极撤机,一定程度抑制膈肌萎缩,预防膈肌功能障碍的发生。利用EIT和测量跨肺呼气末压保持肺均一性;使驱动压<15 cmH2O,跨肺驱动压<10~12 cmH2O;同时监测食道压、P0.1和膈肌增厚分数使患者呼气努力保持在正常水平;确保神经呼吸和机械呼吸的同步[44]。
最近的研究发现,比例辅助通气(PAV)和神经调节通气辅助(NAVA),提供与患者努力成比例的压力辅助,使通气能够更好地由患者的大脑控制,避免过度辅助和辅助不足,改善患者与呼吸机的相互作用,并提供保护性通气[45]。
2018年PADIS指南中声明,成人ICU患者的疼痛管理应以常规疼痛评估为指导,在考虑使用镇静剂之前应治疗疼痛,从而最大限度地降低过度镇静的风险。阿片类药物目前被临床实践指南推荐为第一种基于镇痛的方法以促进机械通气的药物,其与人机不同步化发生率较少相关,因此可能是理想的首选药物。同时,PADIS指南建议危重、机械通气的成人患者使用轻度镇静(RASS评分为-2至+1分),可缩短拔管时间,降低气切率。此外,在ICU中监测呼吸驱动和努力,使其维持在平衡状态有助于膈肌保护性通气的镇静策略[46, 47]。
迄今为止暂无明确的药物被证实可有效预防或治疗VIDD,但一些药物具有较好的前景,需要进一步研究。
有多项动物机械通气研究中表明机械通气可诱导体内氧化应激反应增强,是引起VIDD的关键因素之一。同时证明补充抗氧化剂可降低膈肌损伤,是治疗VIDD的一种潜在措施。有研究在SD大鼠模型中给予抗氧化剂可减轻机械通气对膈肌收缩功能及蛋白水解的有害影响。另一项动物研究表明黄嘌呤氧化酶是一种潜在的超氧化物来源,在机械通气期间膈肌中也有上调,抑制它可以适度改善横膈肌收缩力。同时也有荟萃分析表明,抗氧化剂对危重症患者有益[48]。
茶碱已被证明具有多种药理作用,如扩张支气管、抗炎、刺激呼吸和呼吸神经元网络。此外,茶碱也是一种肌肉激动剂,可提高神经肌肉疾病患者的耐力和握力,促进肋间肌、腹横肌、膈肌等呼吸肌活动。Kim等[49]对ICU患者进行了一项有关茶碱治疗的研究,运用超声检查评估膈肌运动,最终研究结果显示茶碱能显著改善VIDD患者的膈肌运动,进一步表明茶碱可能对伴有VIDD的机械通气脱机困难患者具有治疗价值。一项涉及动物和人类的研究证实茶碱可改善长期机械通气患者的呼吸肌力,茶碱处理组最大吸气压力和浅快呼吸指数明显优于对照组[50]。
钙增敏剂可以在不增加能量消耗的情况下提高肌肉收缩力,可更有效地促进早日脱机。左西孟旦是一种临床使用的钙增敏剂,既往研究发现左西孟旦可增强人类膈肌部分纤维的钙敏感性。Doorduin等[51]研究证明左西孟旦可提高人体膈肌的神经机械效率和收缩功能,可能是改善慢性呼吸衰竭患者呼吸肌功能的一种潜在方法。然而,在另一项研究中发现左西孟旦对脱机时的膈肌收缩效率无影响,但其可加强中枢呼吸驱动,表现为潮气量和分钟通气量增加,PaCO2降低,并且使用左西孟旦后未见严重不良事件报告[52]。
此外,糖皮质激素、血管紧张素-(1-7)和神经肌肉阻滞剂等药物以及对蛋白水解系统的干预也在治疗VIDD中具有不同的潜能。
早在1872年,膈神经刺激被定义为生理学上模拟自然呼吸的最佳方法,1995年第一个呼吸起搏器推向市场,从此膈肌起搏成为原发性低通气患者的当代治疗工具。2021年一项研究表明通过起经皮膈肌电刺激诱导膈肌收缩不仅有可能减缓机械通气时膈肌萎缩的速度,而且可能由于增加膈肌厚度。经皮膈神经电刺激是维持膈肌厚度的一种有效且可行的方法,可能是未来预防甚至是治疗VIDD的一种选择。这为在重症监护环境中维持膈肌功能提供了非侵入性系统的可能性,对专业技能的要求明显降低[53]。同时,一项关于食道膈神经刺激的研究证明食道膈神经刺激较常规刺激可增加有效刺激[54]。膈肌电刺激的选择具有多样性,但均对膈肌功能具有保护作用。
也有研究探讨了吸气肌训练对机械通气患者临床结局的影响。最终结果表明在吸气肌训练后最大吸气压和脱机的可能性显著增加,住院时间缩短。2019年澳大利亚的一份指南中明确提出应采用多学科的ICU团队为患者提供吸气肌训练的康复辅助手段。有创机械通气>7天的患者可以在呼吸机依赖阶段或从机械通气脱机时开始吸气肌训练。每天进行一次高强度训练(5组,共6次呼吸,训练负荷为最大吸气压力的50%),并且每天增加强度,不仅可以改善脱机,吸气肌的力量,而且可改善持续7天乃至长时间的机械通气脱机患者的生活质量[55]。另一项包含360名参与者的描述重症监护治疗师国际实践的研究显示63%的ICU患者使用吸气肌训练。吸气肌训练对于在ICU经历长时间机械通气的患者逆转吸气肌无力和改善预后安全有效。在63%进行吸气肌训练的参与者中,只有64%的人定期评估吸气肌力量。因此在实践中应加强吸气肌训练技术的知识掌握,促进该技术的规范使用[56]。
此外,还有研究表示肺康复联合膈肌起搏治疗重症机械通气患者安全有效。其可改善重度机械通气患者膈肌活动能力,减轻膈肌萎缩和变薄,改善呼吸功能,缩短机械通气时间、ICU和总住院天数,提高患者GCS和APACHE II评分[57]。同时,早期康复治疗有利于改善长期机械通气患者的膈肌功能,尽早撤机和拔管[58]。也有研究探讨了吸气肌训练对机械通气患者临床结局的影响。最终结果表明在吸气肌训练后最大吸气压和脱机的可能性显著增加,住院时间缩短。与此同时,针刺治疗、穴位电刺激等在为呼吸机诱导的肌膈肌功能障碍的治疗方面提供了新思路和新方法。
参考文献
[1] TWOSE P, JONES U, WISE M P. Effect of hypercapnia on respiratory and peripheral skeletal muscle loss during critical illness-A pilot study [J]. J Crit Care, 2018, 45: 105-109.
[2] DRES M, GOLIGHER E C, HEUNKS L M A, et al. Critical illness-associated diaphragm weakness [J]. Intensive Care Med, 2017, 43(10): 1441-1452.
[3] JUNG B, SEBBANE M, LE GOFF C, et al. Moderate and prolonged hypercapnic acidosis may protect against ventilator-induced diaphragmatic dysfunction in healthy piglet: an in vivo study [J]. Crit Care, 2013, 17(1): R15.
[4] TELIAS I, BROCHARD L, GOLIGHER E C. Is my patient's respiratory drive (too) high? [J]. Intensive Care Med, 2018, 44(11): 1936-1939.
[5] JABER S, JUNG B, MATECKI S, et al. Clinical review: ventilator-induced diaphragmatic dysfunction--human studies confirm animal model findings! [J]. Crit Care, 2011, 15(2): 206.
[6] REID W D, BELCASTRO A N. Time course of diaphragm injury and calpain activity during resistive loading [J]. Am J Respir Crit Care Med, 2000, 162(5): 1801-1806.
[7] LECRONIER M, JUNG B, MOLINARI N, et al. Severe but reversible impaired diaphragm function in septic mechanically ventilated patients [J]. Ann Intensive Care, 2022, 12(1): 34.
[8] VAN DER PIJL R J, GRANZIER H L, OTTENHEIJM C A C. Diaphragm contractile weakness due to reduced mechanical loading: role of titin [J]. Am J Physiol Cell Physiol, 2019, 317(2): C167-C176.
[9] GRASSI A, FERLICCA D, LUPIERI E, et al. Assisted mechanical ventilation promotes recovery of diaphragmatic thickness in critically ill patients: a prospective observational study [J]. Crit Care, 2020, 24(1): 85.
[10] MARIN-CORRAL J, DOT I, BOGUñA M, et al. Structural differences in the diaphragm of patients following controlled vs assisted and spontaneous mechanical ventilation [J]. Intensive Care Med, 2019, 45(4): 488-500.
[11] OROZCO-LEVI M, LLORETA J, MINGUELLA J, et al. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease [J]. Am J Respir Crit Care Med, 2001, 164(9): 1734-1739.
[12] ITAGAKI T, AKIMOTO Y, NAKANO Y, et al. Relationships between double cycling and inspiratory effort with diaphragm thickness during the early phase of mechanical ventilation: A prospective observational study [J]. PLoS One, 2022, 17(8): e0273173.
[13] GEA J, ZHU E, GáLDIZ J B, et al. [Functional consequences of eccentric contractions of the diaphragm] [J]. Arch Bronconeumol, 2009, 45(2): 68-74.
[14] JANSEN D, JONKMAN A H, VRIES H J, et al. Positive end-expiratory pressure affects geometry and function of the human diaphragm [J]. J Appl Physiol (1985), 2021, 131(4): 1328-1339.
[15] LINDQVIST J, VAN DEN BERG M, VAN DER PIJL R, et al. Positive End-Expiratory Pressure Ventilation Induces Longitudinal Atrophy in Diaphragm Fibers [J]. Am J Respir Crit Care Med, 2018, 198(4): 472-485.
[16] QIAN X, JIANG Y, JIA J, et al. PEEP application during mechanical ventilation contributes to fibrosis in the diaphragm [J]. Respiratory research, 2023, 24(1): 46.
[17] DE OLIVEIRA B, ALJABERI N, TAHA A, et al. Patient-Ventilator Dyssynchrony in Critically Ill Patients [J]. Journal of clinical medicine, 2021, 10(19):4550.
[18] SPINELLI E, MAURI T, BEITLER J R, et al. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions [J]. Intensive Care Med, 2020, 46(4): 606-618.
[19] SKLAR M C, MADOTTO F, JONKMAN A, et al. Duration of diaphragmatic inactivity after endotracheal intubation of critically ill patients [J]. Crit Care, 2021, 25(1): 26.
[20] DRES M, DUBé B, MAYAUX J, et al. Coexistence and Impact of Limb Muscle and Diaphragm Weakness at Time of Liberation from Mechanical Ventilation in Medical Intensive Care Unit Patients [J]. American journal of respiratory and critical care medicine, 2017, 195(1): 57-66.
[21] PEARSON S D, LIN J, STUTZ M R, et al. Immediate Effect of Mechanical Ventilation Mode and Sedative Infusion on Measured Diaphragm Thickness [J]. Ann Am Thorac Soc, 2022, 19(9): 1543-1550.
[22] KRESS J P, POHLMAN A S, O'CONNOR M F, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation [J]. N Engl J Med, 2000, 342(20): 1471-1477.
[23] MURRAY B, SIKORA A, MOCK J R, et al. Reverse Triggering: An Introduction to Diagnosis, Management, and Pharmacologic Implications [J]. Front Pharmacol, 2022, 13: 879011.
[24] BOON A J, HARPER C J, GHAHFAROKHI L S, et al. Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects [J]. Muscle Nerve, 2013, 47(6): 884-889.
[25] CARRILLO-ESPER R, PéREZ-CALATAYUD Á A, ARCH-TIRADO E, et al. Standardization of Sonographic Diaphragm Thickness Evaluations in Healthy Volunteers [J]. Respir Care, 2016, 61(7): 920-924.
[26] TUINMAN P R, JONKMAN A H, DRES M, et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review [J]. Intensive Care Med, 2020, 46(4): 594-605.
[27] WAIT J L, NAHORMEK P A, YOST W T, et al. Diaphragmatic thickness-lung volume relationship in vivo [J]. J Appl Physiol (1985), 1989, 67(4): 1560-1568.
[28] GOLIGHER E C, LAGHI F, DETSKY M E, et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity [J]. Intensive Care Med, 2015, 41(4): 642-649.
[29] DUBé B P, DRES M, MAYAUX J, et al. Ultrasound evaluation of diaphragm function in mechanically ventilated patients: comparison to phrenic stimulation and prognostic implications [J]. Thorax, 2017, 72(9): 811-818.
[30] GOLIGHER E C, DRES M, FAN E, et al. Mechanical Ventilation-induced Diaphragm Atrophy Strongly Impacts Clinical Outcomes [J]. Am J Respir Crit Care Med, 2018, 197(2): 204-213.
[31] VIVIER E, MEKONTSO DESSAP A, DIMASSI S, et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation [J]. Intensive Care Med, 2012, 38(5): 796-803.
[32] UMBRELLO M, FORMENTI P, LONGHI D, et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study [J]. Crit Care, 2015, 19(1): 161.
[33] MATAMIS D, SOILEMEZI E, TSAGOURIAS M, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications [J]. Intensive Care Med, 2013, 39(5): 801-810.
[34] SOILEMEZI E, TSAGOURIAS M, TALIAS M A, et al. Sonographic assessment of changes in diaphragmatic kinetics induced by inspiratory resistive loading [J]. Respirology, 2013, 18(3): 468-473.
[35] STEIER J, KAUL S, SEYMOUR J, et al. The value of multiple tests of respiratory muscle strength [J]. Thorax, 2007, 62(11): 975-980.
[36] AYOUB J, COHENDY R, DAUZAT M, et al. Non-invasive quantification of diaphragm kinetics using m-mode sonography [J]. Can J Anaesth, 1997, 44(7): 739-744.
[37] MAURI T, GRASSELLI G, SURIANO G, et al. Control of Respiratory Drive and Effort in Extracorporeal Membrane Oxygenation Patients Recovering from Severe Acute Respiratory Distress Syndrome [J]. Anesthesiology, 2016, 125(1): 159-167.
[38] LUO Y M, HART N, MUSTFA N, et al. Effect of diaphragm fatigue on neural respiratory drive [J]. J Appl Physiol (1985), 2001, 90(5): 1691-1699.
[39] AGOSTONI E, RAHN H. Abdominal and thoracic pressures at different lung volumes [J]. J Appl Physiol, 1960, 15: 1087-1092.
[40] LAPORTA D, GRASSINO A. Assessment of transdiaphragmatic pressure in humans [J]. J Appl Physiol (1985), 1985, 58(5): 1469-1476.
[41] BELLANI G, MAURI T, COPPADORO A, et al. Estimation of patient's inspiratory effort from the electrical activity of the diaphragm [J]. Crit Care Med, 2013, 41(6): 1483-1491.
[42] BELLANI G, COPPADORO A, POZZI M, et al. The Ratio of Inspiratory Pressure Over Electrical Activity of the Diaphragm Remains Stable During ICU Stay and is not Related to Clinical Outcome [J]. Respir Care, 2016, 61(4): 495-501.
[43] LAVENEZIANA P, ALBUQUERQUE A, ALIVERTI A, et al. ERS statement on respiratory muscle testing at rest and during exercise [J]. Eur Respir J, 2019, 53(6):1801214..
[44] KARAGEORGOS V, PROKLOU A, VAPORIDI K. Lung and diaphragm protective ventilation: a synthesis of recent data [J]. Expert Rev Respir Med, 2022, 16(4): 375-390.
[45] VAPORIDI K. NAVA and PAV+ for lung and diaphragm protection [J]. Curr Opin Crit Care, 2020, 26(1): 41-46.
[46] GOLIGHER E C, JONKMAN A H, DIANTI J, et al. Clinical strategies for implementing lung and diaphragm-protective ventilation: avoiding insufficient and excessive effort [J]. Intensive Care Med, 2020, 46(12): 2314-2326.
[47] DEVLIN J W, SKROBIK Y, GéLINAS C, et al. Executive Summary: Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU [J]. Crit Care Med, 2018, 46(9): 1532-1548.
[48] HEYLAND D K, DHALIWAL R, SUCHNER U, et al. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient [J]. Intensive Care Med, 2005, 31(3): 327-337.
[49] KIM W Y, PARK S H, KIM W Y, et al. Effect of theophylline on ventilator-induced diaphragmatic dysfunction [J]. J Crit Care, 2016, 33: 145-150.
[50] YU T J, LIU Y C, CHU C M, et al. Effects of theophylline therapy on respiratory muscle strength in patients with prolonged mechanical ventilation: A retrospective cohort study [J]. Medicine (Baltimore), 2019, 98(2): e13982.
[51] DOORDUIN J, SINDERBY C A, BECK J, et al. The calcium sensitizer levosimendan improves human diaphragm function [J]. Am J Respir Crit Care Med, 2012, 185(1): 90-95.
[52] ROESTHUIS L, VAN DER HOEVEN H, SINDERBY C, et al. Effects of levosimendan on respiratory muscle function in patients weaning from mechanical ventilation [J]. Intensive Care Med, 2019, 45(10): 1372-1381.
[53] SOTáK M, ROUBíK K, HENLíN T, et al. Phrenic nerve stimulation prevents diaphragm atrophy in patients with respiratory failure on mechanical ventilation [J]. BMC Pulm Med, 2021, 21(1): 314.
[54] KAUFMANN E M, KRAUSE S, GEISSHUESLER L, et al. Feasibility of transesophageal phrenic nerve stimulation [J]. Biomed Eng Online, 2023, 22(1): 5.
[55] BISSETT B, LEDITSCHKE I A, GREEN M, et al. Inspiratory muscle training for intensive care patients: A multidisciplinary practical guide for clinicians [J]. Aust Crit Care, 2019, 32(3): 249-255.
[56] DA SILVA GUIMARãES B, DE SOUZA L C, CORDEIRO H F, et al. Inspiratory Muscle Training With an Electronic Resistive Loading Device Improves Prolonged Weaning Outcomes in a Randomized Controlled Trial [J]. Crit Care Med, 2021, 49(4): 589-597.
[57] LIU Z B, WANG L Y, ZHAO L, et al. Clinical effect of pulmonary rehabilitation combined with diaphragm pacemaker therapy in the treatment of severely ill patients with mechanical ventilation [J]. Int J Rehabil Res, 2022, 45(3): 195-200.
[58] DONG Z, LIU Y, GAI Y, et al. Early rehabilitation relieves diaphragm dysfunction induced by prolonged mechanical ventilation: a randomised control study [J]. BMC pulmonary medicine, 2021, 21(1): 106.
作者简介
浙江大学医学院附属邵逸夫医院呼吸治疗科呼吸治疗师,硕士
参与发表SCI论文3篇
参与省级课题1项,市级课题2项
江叶
浙江大学医学院附属邵逸夫医院呼吸治疗科肺功能及心肺运动组长
擅长心肺运动评估及呼吸康复指导训练
参与国家自然科学基金项目1项,参编、参译专业书籍3部
发表多篇SCI文章
钱晓丽
浙江大学医学院附属邵逸夫医院呼吸治疗科呼吸治疗师,硕士
以第一作者及共同第一作者发表SCI论文3篇
参与国家级课题1项、省级课题1项、市级课题1项
声明:本文仅用于学术内容的探讨和交流,不用于任何商业和推广,亦不作为最终的临床决策。临床实践需根据患者的具体情况选择适宜的处理措施。
 后可发表评论
后可发表评论



友情链接
联系我们
 公众号
公众号
 客服微信
客服微信