登录方式
方式一:
PC端网页:www.rccrc.cn
输入账号密码登录,可将此网址收藏并保存密码方便下次登录
方式二:
手机端网页:www.rccrc.cn
输入账号密码登录,可将此网址添加至手机桌面并保存密码方便下次登录
方式三:
【重症肺言】微信公众号
输入账号密码登录
注:账号具有唯一性,即同一个账号不能在两个地方同时登录。
临床中,针对细菌感染和真菌感染的治疗关注点有很大不同。对于细菌感染,临床医生可能更多关注在较多的病原菌中及其耐药性分析,而对于真菌感染,病原菌种类有限,可选的药物种类也较少,且耐药问题及关注程度也远不及细菌。在真菌药物治疗方面,最重要的关注点可能是抗真菌药物的PK/PD特点。导致抗真菌治疗效果欠佳甚至治疗失败的原因,可能是药物暴露量不足或药物在感染局部的暴露量不足,以及患者无法耐受。这也是我们在抗真菌治疗中面临的挑战之一。因此,我们应更加深入地了解抗真菌药物的药动学特点及其对疗效和安全性的影响。本文主要阐述唑类抗真菌药的药动学特点及其临床应用。

表1 三唑类药物药动学特点
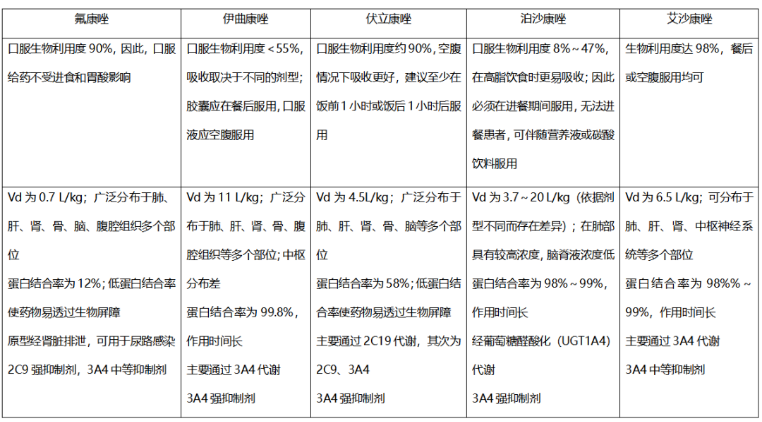
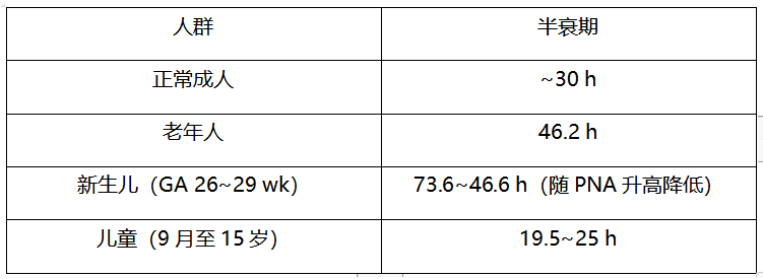
1. 氟康唑
表2 氟康唑在不同人群中的半衰期

2. 伏立康唑
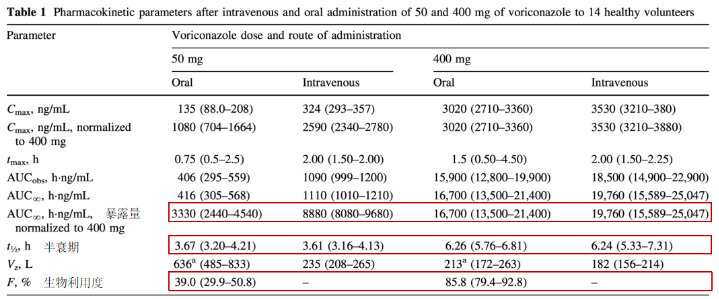
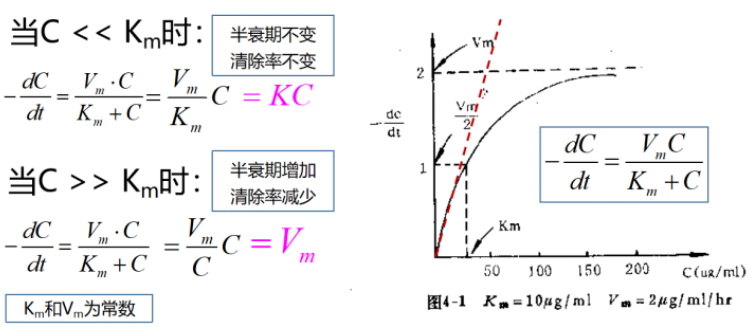
3. 泊沙康唑
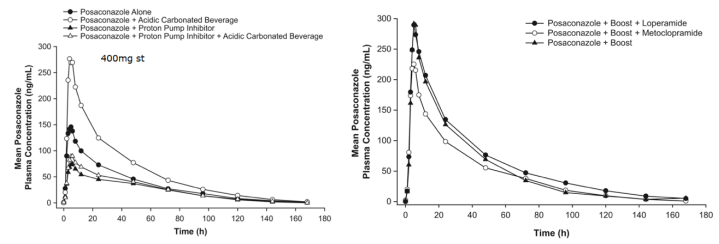
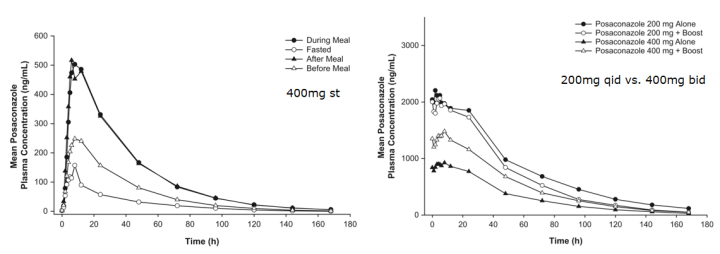
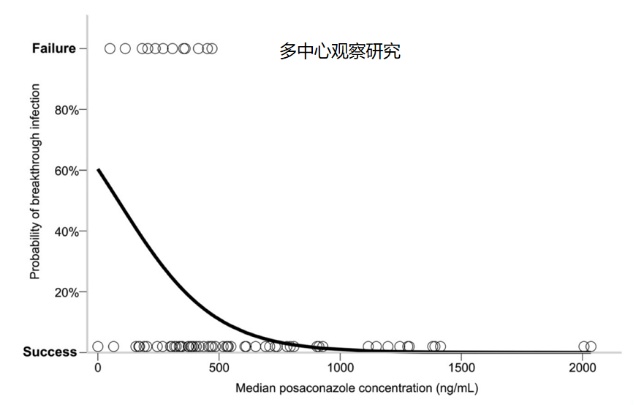
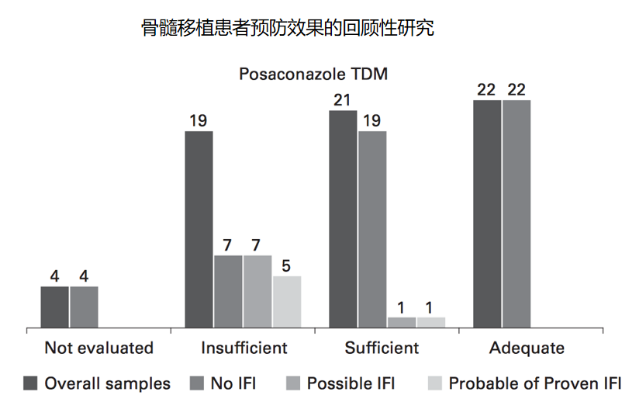
4. 艾沙康唑
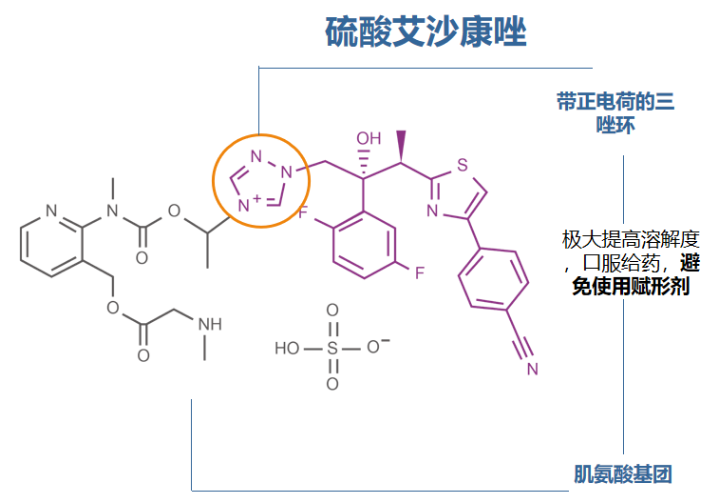
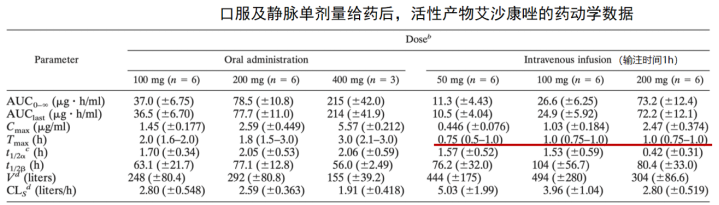
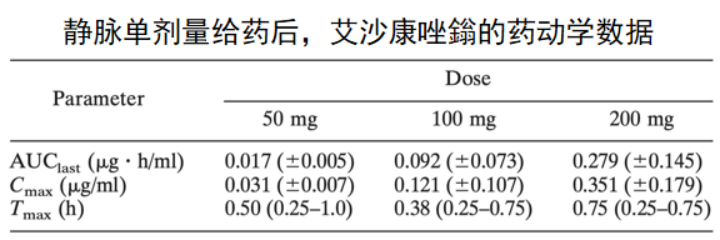
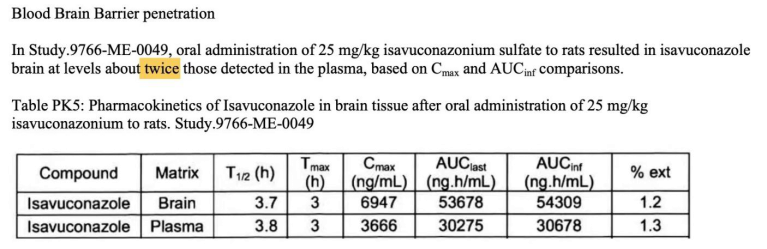

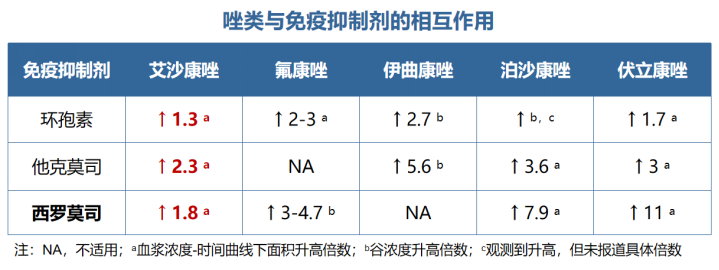

唑类抗真菌药在药动学方面各有特点,具有较大的差异。伏立康唑重点关注在代谢方面的个体差异,泊沙康唑重点关注影响吸收的因素,而艾沙康唑无论在代谢还是吸收方面都具有较好的药动学特点,所以其个体差异比较少,也不需要常规进行血药浓度监测。在治疗中需要考虑不同人群特点,保证疗效及安全性。
参考文献
[1] Hohmann N, Kocheise F, Carls A, et al. Dose-Dependent Bioavailability and CYP3A Inhibition Contribute to Non-Linear Pharmacokinetics of Voriconazole[J]. Clin Pharmacokinet, 2016, 55(12):1535-1545.
[2] Jin H, Wang T, Falcione B A, et al. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis[J]. J Antimocrob Chemother, 2016, 71(7):1772-1785.
[3] Park W B, Kim N H, Kim K H, et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial[J]. Clin Infect Dis, 2012, 55(8):1080-1087.
[4] Krishna G, Moton A, Ma L, et al. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers[J]. Antimicrob Agents Chemother, 2009, 53(3):958-966.
[5] Dolton M J, Brüggemann R J M, Burger D M, et al. Understanding variability in posaconazole exposure using an integrated population pharmacokinetic analysis[J]. Antimicrob Agents Chemother, 2014, 58(11):6879-6885.
[6] Ullmann A J, Cornely O A, Burchardt A, et al. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection[J]. Antimicrob Agents Chemother, 2006, 50(2):658-666.
[7] Dolton M J, Ray J E, Chen S C-A, et al. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration[J]. Antimicrob Agents Chemother, 2012, 56(11):5503-5510.
[8] Greco R, Barbanti M C, Lupo Stranghellini M T, et al. Coadministration of posaconazole and sirolimus in allogeneic hematopoietic stem cell transplant recipients[J]. Bone Marrow Transplant, 2016, 51(7):1022-1024.
[9] Schmitt-Hoffmann A, Roos B, Maares J, et al. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers[J]. Antimicrob Agents Chemother, 2006, 50(1):286-293.
[10] Schmitt-Hoffmann A, Roos B, Heep M, et al. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers[J]. Antimicrob Agents Chemother, 2006, 50(1):279-285.
[11] Kovanda L L, Maher R, Hope W W, et al. Isavuconazonium sulfate: a new agent for the treatment of invasive aspergillosis and invasive mucormycosis[J]. Expert Rev Clin Pharmacol, 2016, 9(7):887-897.
[12] Schmitt-Hoffmann A-H, Kato K, Townsend R, et al. Tissue Distribution and Elimination of Isavuconazole following Single and Repeat Oral-Dose Administration of Isavuconazonium Sulfate to Rats[J]. Antimicrob Agents Chemother, 2017, 61(12):e01292-17.
[13] Groll A H, Desai A, Han D, et al. Pharmacokinetic Assessment of Drug-Drug Interactions of Isavuconazole With the Immunosuppressants Cyclosporine, Mycophenolic Acid, Prednisolone, Sirolimus, and Tacrolimus in Healthy Adults[J]. Clin Pharmacol Drug Dev, 2017, 6(1):76-85.
专家简介

中日友好医院主管药师,抗感染临床药师,信息药师
药事会秘书,抗菌药物管理小组秘书
卫健委临床药师培训基地带教师资
耶鲁纽黑文医院访问学者
中国医疗保健国际交流促进会临床微生物与感染分会青年委员
中国研究型医院学会药物评价专业委员会青年委员
北京药学会感染性疾病药物治疗与风险管理专业委员会委员
 后可发表评论
后可发表评论



友情链接
联系我们
 公众号
公众号
 客服微信
客服微信