登录方式
方式一:
PC端网页:www.rccrc.cn
输入账号密码登录,可将此网址收藏并保存密码方便下次登录
方式二:
手机端网页:www.rccrc.cn
输入账号密码登录,可将此网址添加至手机桌面并保存密码方便下次登录
方式三:
【重症肺言】微信公众号
输入账号密码登录
注:账号具有唯一性,即同一个账号不能在两个地方同时登录。
作者:刘凯雄
单位:福建医科大学附属第一医院呼吸与危重症医学科
01
重症PJP患者的病死率在50%以上。德国ICU住院PJP患者病死率为58%,其中实体器官移植患者病死率更是高达78.9%[5]。中日友好医院与北京协和医院联合开展的一项回顾性研究发现,ICU中非HIV重症PJP患者的病死率高达75.6%,其中79.2%的患者为结缔组织病,而此类患者通常病情都较重;在死亡患者中,纵隔气肿比例高达23.17%[6]。
02
1909年,Chagas首次在豚鼠中发现肺孢子菌,认为是克氏锥虫一种。1910年Carini在大鼠肺组织中发现肺孢子菌。1912年,Delanoe夫妇将其鉴定为新的原虫,并命名卡氏肺孢子/囊虫(Pneumocystis carinii)。1976年,Delanoe提议将其改为耶氏肺孢子菌(Pneumocystis jirovecii,PJ),以纪念捷克寄生虫学家耶诺维奇(Jirovecii)。1988年,Edman发现其核糖体RNA与真菌类似,提出分子水平PJ属于真菌。2001年,在机会性原生生物国际研讨会上,正式将PJP代替PCP,之后文献越来越多采用PJP。
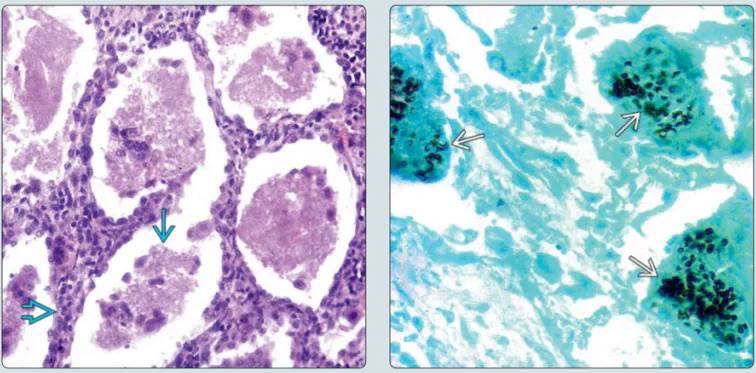
PJ主要有包囊(感染型)和滋养体(繁殖型)两种形态,滋养体为可变多形体,有细足和伪足形成,类似阿米巴;包囊呈圆形,直径4~6 μm,囊壁内含有囊内小体(或称子孢子),完全成熟的包囊内一般为8个,包囊是重要的诊断形态。PJ多寄生于肺泡腔(滋养体、包囊前期和包囊期),成熟包囊进入肺泡后破裂,囊内小体脱囊后发育为滋养体,滋养体紧贴肺泡上皮寄生,增殖,包囊多位于肺泡中央[7]。PJ可以通过气溶胶和人传人进行传播,ICH为PJ的主要宿主[8]。
03
04
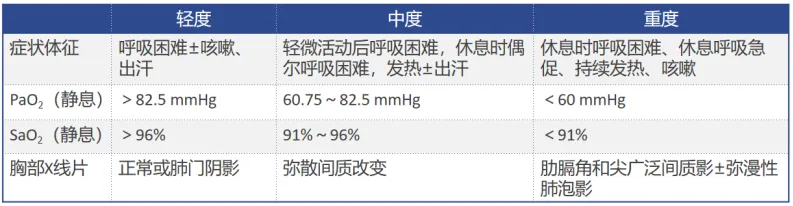
在高度怀疑PJP的情况下,G试验有助于明确诊断。若G试验结果≥80 pg/ml,诊断PJP的敏感性为69.8%,特异性为81.2%,阳性预测值为34.6%,阴性预测值为95.2%。若G试验结果≥200 pg/ml联合PCR阳性,诊断PJP的敏感性为70%,特异性为100%,阳性预测值为100%,阴性预测值为52.0%[23]。最新荟萃分析纳入23项研究,显示G试验诊断PJP的敏感性为91%,特异性为79%,其中HIV患者敏感性为94%,非HIV敏感性为86%;若G试验阴性,PJP可能性小[24]。对于送检的血标本或呼吸道标本,也可以采用PCR,每1 ml送检样本超过1450个病原体,PCR的诊断价值较高。不同研究显示血清PCR的敏感性>70%[25, 26]。
05
06
北京协和医院的一项研究报道,NPPV在重症PJP患者中使用率低,即使使用,其失败率也高达83.3%[31]。因此对于重症PJP患者,在有插管指征的情况下一定要尽早插管,以免延误病情。目前有关ECMO在重症PJP患者中的应用研究例数较少。一项研究纳入了接受ECMO治疗的31例PJP患者,其中6例HIV患者,其他为接受免疫抑制治疗患者,总体存活率为31%。该研究发现,清醒ECMO的临床获益优于插管ECMO[32]。较多患者在撤离ECMO后死亡,因此对于接受ECMO治疗的PJP患者抗PJ的疗程可能需要延长。
德国一项为期10年的单中心回顾性研究中共有18例PJP患者接受ECMO治疗,结果显示,非HIV PJP患者的总体存活率仅为8.3%,而HIV PJP患者的存活率为50%[33],1/3患者成功撤离ECMO后仍有75%死亡。因此,ECMO治疗重症PJP仍需要更多的经验积累。
在PJP的治疗药物中,复方磺胺甲噁唑为首选。欧洲白血病抗感染委员会(ECIL)关于非HIV感染血液科患者PJP治疗指南[34]推荐的一线治疗为磺胺甲噁唑+甲氧苄啶(5:1)TMP/SMX(首选治疗),TMP推荐剂量为15~20 mg/kg,SMX剂量为75~100 mg/kg;建议早期静脉或口服、足疗程用药。
目前卡泊芬净在PJP治疗中的地位多为体外研究文献支持,人体研究通常为小样本观察性研究,临床上卡泊芬净多与复方磺胺甲噁唑联用。《抗菌药物超说明书用法专家共识》也提到卡泊芬净联合磺胺甲噁唑/甲氧苄啶可能是治疗PJP的有效方案之一[35]。PJP在滋养体期没有β-1,3葡聚糖,因此卡泊芬净对于滋养体期的PJ是无效的。PJP合并曲霉的感染发生率较高。国内一些大型医疗中心卡泊芬净的使用率为40%~50%。武汉大学人民医院对于肾移植后PJP的治疗,100%采用卡泊芬净联合磺胺甲噁唑/甲氧苄啶[36]。
磺胺类药物的母体为对氨基苯磺酰胺(磺胺,SN),最早是合成偶氮染料的中间体。1908年已经被合成,但当时无人注意到其医疗价值。直至1932年,德国多马克发现含有磺胺结构片段的磺胺柯定(盐酸盐为百浪多息,KI-730,世界上第一款商业化的合成抗菌药),可使鼠、兔免受链球和葡萄球菌的感染,于次年报告了第一例用百浪多息治疗葡萄球菌引起的败血症,并获得1939年的诺贝尔生理学和医学奖,引起了世界范围的广泛关注。
复方磺胺甲噁唑是磺胺甲噁唑和甲氧苄啶(5:1)组成的复方制剂,磺胺甲噁唑与PABA结构类似,结合二氢叶酸合成(DHPS)基因位点从而抑制DHPS的生成。甲氧苄啶抑制二氢叶酸还原酶,可使细菌的四氢叶酸的合成受到双重阻断,使磺胺药的抗菌作用增强数倍至数十倍,甚至出现杀菌作用,并延缓耐药性的产生。这两个组分构成双重阻断叶酸代谢,疗效大大增强。复方磺胺甲噁唑静脉制剂的达峰速度快,药物浓度高,对于病情危重、无法口服药物的患者,可采用静脉给药。磺胺甲噁唑和甲氧苄啶的半衰期一致,协同性较好。
复方磺胺甲噁唑的副作用包括:①皮疹:发生率低,一旦发生皮疹,应立即停用。严重皮肤不良反应包括史蒂文斯-约翰逊综合征(SJS)、中毒性表皮坏死松解(TEN)和系统症状药疹(DRESS);②血小板减少:多为免疫介导,停药1周后多可恢复;③肝毒性:免疫介导,与基因有关,HIV感染发生率为16.4%;④骨髓抑制;⑤磺胺结晶阻塞肾小管:用药时需充分水化,保证尿液>1500 ml,尿pH>7.15。
如何安全地静脉滴注复方磺胺甲噁唑?稀释和静脉滴注要点包括:①稀释25倍,4 h之内使用;②缓慢静滴,至少滴注60~90 min;③5%葡萄糖稀释优于0.9%氯化钠(更大的溶解度、更低的渗透压)。若患者重症,限水,建议至少稀释到150 ml,静脉滴注比较安全。肌酐清除率>30 ml/min,复方磺胺甲噁唑可以正常剂量使用;肌酐清除率在15~30 ml/min,减半使用;肌酐清除率<15 ml/min,一般不推荐使用。
07

[1] Patel M, Chen J, Kim S, et al. Analysis of MarketScan Data for Immunosuppressive Conditions and Hospitalizations for Acute Respiratory Illness, United States[J]. Emerg Infect Dis, 2020, 26(8):1720-1730.
[2] Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients[J]. Int J Infect Dis, 2016, 46:11-17.
[3] Li Y, Sun B, Tang X, et al. Application of metagenomic next-generation sequencing for bronchoalveolar lavage diagnostics in critically ill patients[J]. Eur J Clin Microbiol Infect Dis, 2020, 39(2):369-374.
[4] Wu X, Li Y, Zhang M, et al. Etiology of Severe Community-Acquired Pneumonia in Adults Based on Metagenomic Next-Generation Sequencing: A Prospective Multicenter Study[J]. Infect Dis Ther, 2020, 9(4):1003-1015.
[5] Schmidt J J, Lueck C, Ziesing S, et al. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years[J]. Crit Care, 2018, 22(1):307.
[6] Weng L, Huang X, Chen L, et al. Prognostic factors for severe Pneumocystis jiroveci pneumonia of non-HIV patients in intensive care unit: a bicentric retrospective study[J]. BMC Infect Dis, 2016, 16(1):528.
[7] Cushion M T, Stringer J R. Stealth and opportunism: alternative lifestyles of species in the fungal genus Pneumocystis[J]. Annu Rev Microbiol, 2010, 64:431-452.
[8] Ponce C A, Gallo M, Bustamante R, et al. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population[J]. Clin Infect Dis, 2010, 50(3):347-353.
[9] Hoving J C, Kolls J K. New advances in understanding the host immune response to Pneumocystis[J]. Curr Opin Microbiol, 2017, 40:65-71.
[10] Messiaen P E, Cuyx S, Dejagere T, et al. The role of CD4 cell count as discriminatory measure to guide chemoprophylaxis against Pneumocystis jirovecii pneumonia in human immunodeficiency virus-negative immunocompromised patients: A systematic review[J]. Transpl Infect Dis, 2017 Apr;19(2). doi: 10.1111/tid.12651.
[11] Grønseth S, Rogne T, Hannula R, et al. Epidemiological and clinical characteristics of immunocompromised patients infected with Pneumocystis jirovecii in a twelve-year retrospective study from Norway[J]. BMC Infect Dis, 2021, 21(1):659.
[12] Schmidt J J, Lueck C, Ziesing S, et al. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years[J]. Crit Care, 2018, 22(1):307.
[13] Roux A, Canet E, Valade S, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France[J]. Emerg Infect Dis, 2014, 20(9):1490-1497.
[14] Guo F, Chen Y, Yang SL, et al. Pneumocystis pneumonia in HIV-infected and immunocompromised non-HIV infected patients: a retrospective study of two centers in China[J]. PLoS One, 2014, 9(7):e101943.
[15] Li L, Hsu S H, Gu X, et al. Aetiology and prognostic risk factors of mortality in patients with pneumonia receiving glucocorticoids alone or glucocorticoids and other immunosuppressants: a retrospective cohort study[J]. BMJ Open, 2020, 10(10):e037419.
[16] Huang L, Fu Q, Ye Y, et al. High incidence and mortality of Pneumocystis jirovecii infection in anti-MDA5-antibody-positive dermatomyositis: experience from a single center[J]. Arthritis Res Ther, 2021, 23(1):232.
[17] Iriart X, Challan Belval T, Fillaux J, et al. Risk factors of Pneumocystis pneumonia in solid organ recipients in the era of the common use of posttransplantation prophylaxis[J]. Am J Transplant, 2015, 15(1):190-199.
[18] Azoulay E, Roux A, Vincent F, et al. A Multivariable Prediction Model for Pneumocystis jirovecii Pneumonia in Hematology Patients with Acute Respiratory Failure[J]. Am J Respir Crit Care Med, 2018, 198(12):1519-1526.
[19] González-Lara M F, Wisniowski-Yáñez A, Pérez-Patrigeon S, et al. Pneumocystis jiroveci pneumonia and GATA2 deficiency: Expanding the spectrum of the disease[J]. J Infect, 2017, 74(4):425-427.
[20] Siddiqi A E, Liu A Y, Charville G W, et al. Disseminated Pneumocystis jirovecii Infection with Osteomyelitis in a Patient with CTLA-4 Haploinsufficiency[J]. J Clin Immunol, 2020, 40(2):412-414.
[21] Roux A, Canet E, Valade S, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France[J]. Emerg Infect Dis, 2014, 20(9):1490-1497.
[22] Guo F, Chen Y, Yang S L, et al. Pneumocystis pneumonia in HIV-infected and immunocompromised non-HIV infected patients: a retrospective study of two centers in China[J]. PLoS One, 2014, 9(7):e101943.
[23] Morjaria S, Frame J, Franco-Garcia A, et al. Clinical Performance of (1,3) Beta-D Glucan for the Diagnosis of Pneumocystis Pneumonia (PCP) in Cancer Patients Tested With PCP Polymerase Chain Reaction[J]. Clin Infect Dis, 2019, 69(8):1303-1309.
[24] Del Corpo O, Butler-Laporte G, Sheppard D C, et al. Diagnostic accuracy of serum (1-3)-β-D-glucan for Pneumocystis jirovecii pneumonia: a systematic review and meta-analysis[J]. Clin Microbiol Infect, 2020, 26(9):1137-1143.
[25] Mühlethaler K, Bögli-Stuber K, Wasmer S, et al. Quantitative PCR to diagnose Pneumocystis pneumonia in immunocompromised non-HIV patients[J]. Eur Respir J, 2012, 39(4):971-978.
[26] Tia T, Putaporntip C, Kosuwin R, et al. A highly sensitive novel PCR assay for detection of Pneumocystis jirovecii DNA in bronchoalveloar lavage specimens from immunocompromised patients[J]. Clin Microbiol Infect, 2012, 18(6):598-603.
[27] Walker C M, Abbott G F, Greene R E, et al. Imaging pulmonary infection: classic signs and patterns[J]. AJR Am J Roentgenol, 2014, 202(3):479-492.
[28] Lu C L, Hung C C. Reversible cystic lesions of Pneumocystis jirovecii pneumonia[J]. Am J Respir Crit Care Med, 2012, 185(6):e7-e8.
[29] Yu Q, Jia P, Su L, et al. Outcomes and prognostic factors of non-HIV patients with pneumocystis jirovecii pneumonia and pulmonary CMV co-infection: A Retrospective Cohort Study[J]. BMC Infect Dis, 2017, 17(1):392.
[30] Ding L, Huang H, Wang H, et al. Adjunctive corticosteroids may be associated with better outcome for non-HIV Pneumocystis pneumonia with respiratory failure: a systemic review and meta-analysis of observational studies[J]. Ann Intensive Care, 2020, 10(1):34.
[31] 黄絮, 翁利, 易丽, 等. 非HIV免疫抑制患者肺孢子菌肺炎合并急性呼吸衰竭的临床特征[J]. 中华医学杂志, 2016, 96(38):3057-3061.
[32] Stahl K, Schenk H, Seeliger B, et al. Extracorporeal membrane oxygenation for acute respiratory distress syndrome due to Pneumocystis pneumonia[J]. Eur Respir J, 2019, 54(3):1900410.
[33] Rilinger J, Staudacher D L, Rieg S, et al. Extracorporeal membrane oxygenation in Pneumocystis jirovecii pneumonia: outcome in HIV and non-HIV patients[J]. Crit Care, 2019, 23(1):356.
[34] Maschmeyer G, Helweg-Larsen J, Pagano L, et al. ECIL guidelines for treatment of Pneumocystis jirovecii pneumonia in non-HIV-infected haematology patients[J]. J Antimicrob Chemother, 2016, 71(9):2405-2413.
[35] 中国医药教育协会感染疾病专业委员会, 中华结核和呼吸杂志编辑委员会, 中国药学会药物临床评价研究专业委员会. 抗菌药物超说明书用法专家共识[J]. 中华结核和呼吸杂志, 2015, 38(6):410-444.
[36] Zou J, Wang T, Qiu T, et al. Single-center retrospective analysis of Pneumocystis jirovecii pneumonia in patients after deceased donor renal transplantation[J]. Transpl Immunol, 2022, 72:101593.
*本文根据“呼吸危重症菁英秀”第二十七期专题视频整理,感谢刘凯雄医师予以审核。
 后可发表评论
后可发表评论



友情链接
联系我们
 公众号
公众号
 客服微信
客服微信